Research Interests
Publications
The High Mobility Group A (HMGA) proteins are a
class of nonhistone chromatin binding proteins that mediate neoplastic
transformation. The 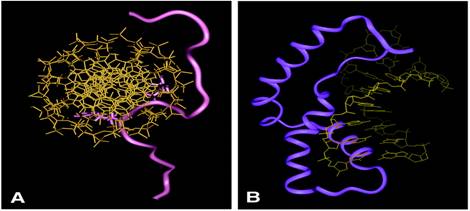 protein's ability to modulate chromatin structure and mediate neoplastic
transformation. This work is currently
funded by the National Science Foundation and will provide essential
information regarding the importance of post-translational modification in
regulating the function of various nuclear proteins. It is likely that understanding of the protein's mechanism in cancer
initiation will facilitate the development of effective treatment
therapies.
protein's ability to modulate chromatin structure and mediate neoplastic
transformation. This work is currently
funded by the National Science Foundation and will provide essential
information regarding the importance of post-translational modification in
regulating the function of various nuclear proteins. It is likely that understanding of the protein's mechanism in cancer
initiation will facilitate the development of effective treatment
therapies.
Students in my lab typically major in biology and/or chemistry and receive extensive training in molecular biology techniques such as cloning, mutagenesis, protein purification, transfection, tissue culture, and electrophoretic mobility shift assays. Several students are also evaluating the DNA binding properties of wild-type and mutant HMGA1 using fluorescence spectroscopy.