CHEM106 Review Questions
-
Draw the molecular structure showing all bonds and charges for the three
most concentrated forms of phenylalanine at pH's of 1.0, 5.0, 7.4, and
11.5. For each pH rank order the relative concentrations of these
forms (you should know the structures for the following amino acids:
Nonpolar - glycine, alanine, valine, leucine, isoleucine, phenylalanine;
Polar - serine, tyrosine, cysteine; Acidic - aspartic acid, glutamic acid;
and Basic - lysine, arginine)
-
Draw the structure of a saturated fatty acid and clearly show the atoms
having significant partial charges.
-
Compare the boiling points and intermolecular forces present for these
pure substances: diethylether, glycerol, and propane; for each of these
three substances, draw diagrams that clearly illustrate the intermolecular
forces.
-
Draw the structure of an aspirin molecule and show the points of hydrogen
bond donors and acceptors.
-
Molecular oxygen has a bond energy of 495 kJ/mole. Calculate the
fraction of molecules that would have a kinetic energy equal to or greater
than this at a temperature of 10,000 K.
-
Use potential and kinetic energy concepts to explain why the moon does
not have an atmosphere; relate this to the vapor pressure of a substance.
-
Draw a diagram illustrating energy changes for an enzyme-catalyzed reaction
and compare to that of an uncatalyzed reaction. Draw diagrams to
clearly show the underlying interactions responsible for the difference.
Explain specifically why enzyme-catalyzed reactions occur faster.
-
Discuss the effect of pH changes and temperature changes on the rate of
enzyme catalyzed reactions; clearly explain the underlying reasons for
each of these.
-
What must happen for chemical reactions to occur?
-
Draw the reaction for the formation of a triglyceride; clearly show the
mechanism of reaction (should be able to do this for the formation of
a dipeptide, a phospholipid, a dinucleotide, and an ester)
-
Use the below structure for glucose to clearly show how it forms a cyclic
structure:
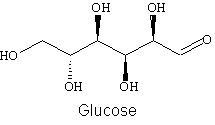
-
Draw the molecular structure (showing all bonds and charges at physiological
pH) of a phospholipid that has the following subunits: an unsaturated fatty
acid, a saturated fatty acid, choline, and phosphate.
-
Draw a diagram that clearly shows, on an atomic level, the interactions
responsible for protein alpha helix and beta sheet secondary structures.
-
Clearly illustrate the surfactant properties of phospholipids by drawing
a molecular structure under physiological pH conditions and by explaining
how this structure accounts for its surfactant properties.
-
Draw a structure for the cholesterol molecule and show its role in cell
membranes.
-
Explain how a set of enzyme kinetic data can be analyzed using the Lineweaver-Burke
model to determine turnover number, KM, and maximal reaction
velocities; explain the physical significance of KM.
-
The pKa of flouroacetic acid is 2.6; for a pH of 5.0, calculate the relative
amounts of the two forms present and draw the structure for each.
-
What are globular proteins and describe the expected orientation of amino
acid side groups in globular proteins that are dissolved in water.
-
Draw the structure of the dipeptide formed from the amino acids cysteine
and alanine. For the peptide bond draw an appropriate resonance structure
and explain the geometry associated with the peptide bond.
-
A given first order reaction had rate constants of 25 sec-1
and 250 sec-1 at temperatures of 298K and 1000K respectively.
Calculate the activation energy for this reaction. Comment on how
the ratio of these two rate constants would change if the reaction had
a lower activation energy and explain why.
-
Given a chemical reaction A + B + C --> Products and the following kinetic
data obtained for this reaction at a temperature of 298K:
|
Run
|
[A]
|
[B]
|
[C]
|
Rate (M/sec)
|
|
1
|
0.151 M
|
0.213 M
|
0.398 M
|
0.480 M/sec
|
|
2
|
0.251 M
|
0.105 M
|
0.325 M
|
0.356 M/sec
|
|
3
|
0.151 M
|
0.213 M
|
0.525 M
|
1.102 M/sec
|
|
4
|
0.151 M
|
0.250 M
|
0.480 M
|
0.988 M/sec
|
-
Calculate the rate law for this reaction
-
Calculate the value of the rate constant k
-
Calculate the overall rate of reaction if each reactant had an initial
concentration of 0.100 M.
-
Two enzyme-catalyzed reactions had KM's of 1.4 x 10-5
and 2.8 x 10-8 M respectively. Comment on the relative
stability of the enzyme-substrate complexes in these two reactions and
provide a plausible explanation for this difference.
-
Draw the structure for pentanoic acid and design a protein "active site"
that would effectively interact with pentanoic acid by clearly showing
how at least five amino acid side-chains that could effectively interact
with the pentanoic acid substrate.
-
Clearly show how COX-2 inhibitors affect the body's inflammatory process
by illustrating the major pathways and by showing their mechanism of action.
-
Draw naproxen's structure at physiological pH and clearly show sites of
hydrogen bonding donors and acceptors. Clearly show the COX-2 amino
acid side chains you would expect to interact with naproxen at the active
site and clearly illustrate these specific interactions (see handout on
COX-2 active site structure).
-
Show the reaction that occurs when aspirin interacts with the COX-1 enzyme
and explain how this interaction is different from that observed for other
nonsteroidal anti-inflammatories.
-
Explain the differences between COX-1 and COX-2 enzymes and relate these
to the molecular structural differences between COX-2 selective inhibitors
and nonspecific COX inhibitors
-
Compare the anti-inflammatory mechanism of action of steroids and of COX-2
inhibitors.
-
Discuss the Lewis Acid and Lewis Base characteristics of zinc fingers
-
Show the reaction that joins two nucleotides and the mechanism for how
it occurs
-
Draw the predominant structure for the phosphate ion at physiological pH
(look up the three pKa's for phosphoric acid to answer this); relate this
to the predominant structure for a nucleotide.
-
A recent French patent claimed intellectual property rights to a new compound
found to have acetylcholinesterase inhibitory potency IC50 (30 nM) in comparison
with the values of other commercially available compounds (galanthamine
IC50 360 nM; tacrine IC50 80 nM) that are being used to treat Alzheimers.
Answer these questions:
-
Outline the mechanism of action and explain why these drugs are partially
effective at delaying the symptoms of AD.
-
Draw a graph that clearly illustrates the IC50's for all three as a function
of enzyme (acetylcholinesterase) activity.
-
Relate IC50 values to the respective Michaelis constants KM's.
-
If a nerve cell had intracellular and extracellular calcium ion concentrations
of 1.5 micomolar and 2050 micromolar respectively, calculate the change
in Gibbs Free Energy that would be required to transfer one millionth of
a mole of calcium ions from the inside of the cell to the outside of the
cell. Assuming that the membrane voltage gradient is solely determined
by the relative calcium concentrations, calculate the membrane potential
needed to maintain this gradient. Draw a diagram and clearly show
how this voltage allows the maintenance of this concentration gradient.
-
Calculate the change in Gibb's Free Energy required to move a millimole
of positive ions out of a cell across a 70 mV membrane gradient.
-
Outline the molecular mechanism of action for local anesthetics and identify
two substances that have utlity in that role.
-
Advair, a combination of a corticosteroid and a bronchodilator, is often
prescribed to treat asthma. Outline what asthma is, the role of the
coroticosteroid in treating asthma, and the norepinephrine receptor targeted
by the bronchodilator. For the latter, describe the specific molecular
mechanism of action that causes bronchial dilation.
-
Outline the molecular mechanism for the action of general anesthetics.
-
Discuss what opioid substances are and the effect that they have on humans.
Outline the basic mechanism of action for their therapeutic role.
-
Draw the structure for a GPCR, explain its role, and outline several examples
of these that have been discussed.
-
Discuss the actions of MAO inhibitors and discuss the molecular mechanism
of action through which MAO inhibitors could potentially be used to treat
Parkinson's Disease.
-
Compare the sizes of potassium and sodium ions by using their respective
electron configurations to support your predictions. Explain why
and how potassium ion channels allow the selective passage of K+
ions, but prevent the passage of Na+ ions; while sodium
ion channels allow the passage of Na+ ions, but prevent the
passage of K+ ions.
-
Describe the molecular structure of a potassium ion channel and explain
how it works.
-
Describe how SSRI's are effective in treating depression by explaining
their molecular mechanism of action.
-
Discuss the role of GABA and glycine receptors and clearly outline the
electrochemical basis upon which GABA/glycine agonists affect the nerve
membrane potential and the excitability of the related neuron.
-
Write the chemical name for GABA and show the molecular structure of the
most concentrated form of GABA at physiological pH.
-
Relate the structure of L-DOPA and dopamine to blood-brain barrier solubility;
use differences in molecular structure to explain this variation.
-
For the thermal decomposition of calcium carbonate:
-
Write the reaction that occurs
-
Predict the change in enthalphy and in entropy for this reaction and provide
the key rationale for your answer.
-
Predict (calculate) the temperature conditions under which this reaction
becomes spontaneous.
-
Calculate the equilibrium constants for this reaction at temperatures of
298K and of 2000K.

